Introduction
The Herceptin Biosimilars Market focuses on biologic medicines designed to replicate the clinical efficacy and safety of Herceptin (trastuzumab), a monoclonal antibody therapy used primarily for HER2-positive breast and gastric cancers. Herceptin revolutionized targeted cancer therapy by specifically inhibiting the HER2 receptor, which plays a key role in tumor cell proliferation. Biosimilars of Herceptin offer comparable therapeutic benefits at lower costs, making them essential in improving accessibility to life-saving cancer treatments.
The growing burden of breast and gastric cancers, coupled with government initiatives to enhance access to biologics, has strengthened the market’s importance in modern oncology.
Learn how the Herceptin Biosimilars Market is evolving—insights, trends, and opportunities await. Download report: https://www.databridgemarketresearch.com/reports/global-herceptin-biosimilars-market
The Evolution
The evolution of the Herceptin Biosimilars Market is closely tied to advancements in biologic drug development and regulatory frameworks supporting biosimilar approvals. Herceptin, developed by Genentech and approved by the U.S. FDA in 1998, set a new standard for targeted cancer therapy. As the original product’s patents expired across key markets—beginning in Europe in 2014 and in the United States in 2019—biosimilar manufacturers entered the market with alternatives that met stringent equivalence standards.
Key milestones include the first Herceptin biosimilar approval in Europe in 2017 and subsequent global launches by companies such as Amgen, Mylan, Pfizer, and Samsung Bioepis. These approvals followed extensive clinical trials demonstrating equivalence in pharmacokinetics, efficacy, and immunogenicity.
Over time, the market has evolved from limited regional adoption to widespread acceptance across oncology centers. Technological advancements in cell-line engineering, purification techniques, and analytical characterization have improved biosimilar quality and manufacturing efficiency. The evolution of global regulatory guidelines by the EMA, FDA, and WHO has also enhanced market confidence and paved the way for multiple biosimilar entrants.
Market Trends
The Herceptin Biosimilars Market is characterized by dynamic growth patterns influenced by healthcare economics, oncology treatment protocols, and technological progress. Key trends include:
Rising Adoption in Oncology Care:
Increasing acceptance of biosimilars by oncologists, payers, and healthcare systems is accelerating market growth. Hospitals and clinics are integrating biosimilars into treatment regimens due to proven therapeutic equivalence and lower costs.Expansion of Reimbursement Policies:
Governments and insurance providers are expanding reimbursement coverage for biosimilars, supporting market penetration and patient access.Technological Advancements in Bioprocessing:
The use of advanced biomanufacturing technologies, such as single-use bioreactors and continuous production systems, is improving yield, reducing costs, and enhancing scalability.Globalization of Biosimilar Production:
Manufacturers in Asia-Pacific, particularly in South Korea, India, and China, are investing heavily in biosimilar R&D and export strategies, leading to global market expansion.Strategic Collaborations and Partnerships:
Biopharmaceutical firms are forming alliances for co-development, licensing, and distribution to strengthen geographic presence and regulatory access.Shift Toward Value-based Healthcare:
As healthcare systems emphasize cost-effectiveness and patient outcomes, biosimilars are positioned as strategic assets to reduce expenditure while maintaining therapeutic efficacy.Increased Clinical Awareness and Education:
Educational campaigns targeting clinicians and patients are improving confidence in biosimilar therapies, leading to broader acceptance and use.
Challenges
Despite strong growth potential, the Herceptin Biosimilars Market faces significant challenges affecting scalability and profitability:
Complex Manufacturing Requirements:
Producing biosimilars involves intricate processes, including cell culture, protein folding, and purification. Maintaining batch consistency is a technical challenge.Regulatory Stringency:
Biosimilars require rigorous analytical and clinical evaluations to demonstrate similarity, extending approval timelines and increasing costs.High Development and Production Costs:
The average cost of developing a biosimilar can exceed USD 100 million, creating barriers for small and mid-sized firms.Market Competition:
Intense competition among global players leads to price erosion, affecting profit margins and return on investment.Intellectual Property Barriers:
Legal disputes and secondary patents on manufacturing processes or formulations can delay biosimilar launches.Physician and Patient Hesitancy:
Despite proven efficacy, some healthcare professionals and patients remain cautious about switching from originator biologics to biosimilars.Supply Chain and Distribution Constraints:
The cold-chain logistics required for biologic drugs pose challenges, especially in low-income regions with limited infrastructure.
Market Scope
Segmentation by Type:
Monotherapy Biosimilars
Combination Therapy Biosimilars
Segmentation by Cancer Type:
Breast Cancer
Gastric Cancer
Other HER2-positive Cancers
Segmentation by Distribution Channel:
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
Segmentation by Technology:
Recombinant DNA Technology
Hybridoma Technology
Mammalian Cell Expression Systems
Regional Analysis:
North America:
The United States and Canada dominate due to advanced healthcare infrastructure, favorable reimbursement systems, and early adoption of biosimilars. The region represents a significant share of global revenue, supported by FDA approvals and growing trust among oncologists.Europe:
Europe is a mature market for biosimilars with established regulatory frameworks. Countries such as Germany, France, and the U.K. exhibit strong adoption rates due to supportive government policies and cost-containment initiatives.Asia-Pacific:
The fastest-growing region due to large patient populations, rising cancer prevalence, and strong biosimilar manufacturing capabilities in South Korea, India, and China. Increased government initiatives for affordable oncology care drive market expansion.Latin America:
The market is developing steadily with increasing regulatory approvals and growing healthcare investments in countries like Brazil and Mexico.Middle East & Africa:
Adoption is emerging as healthcare infrastructure improves. Government partnerships and biosimilar imports are enhancing accessibility in the region.
End-User Industries:
Hospitals and Oncology Clinics
Pharmaceutical Companies
Research and Academic Institutions
Market Size and Factors Driving Growth
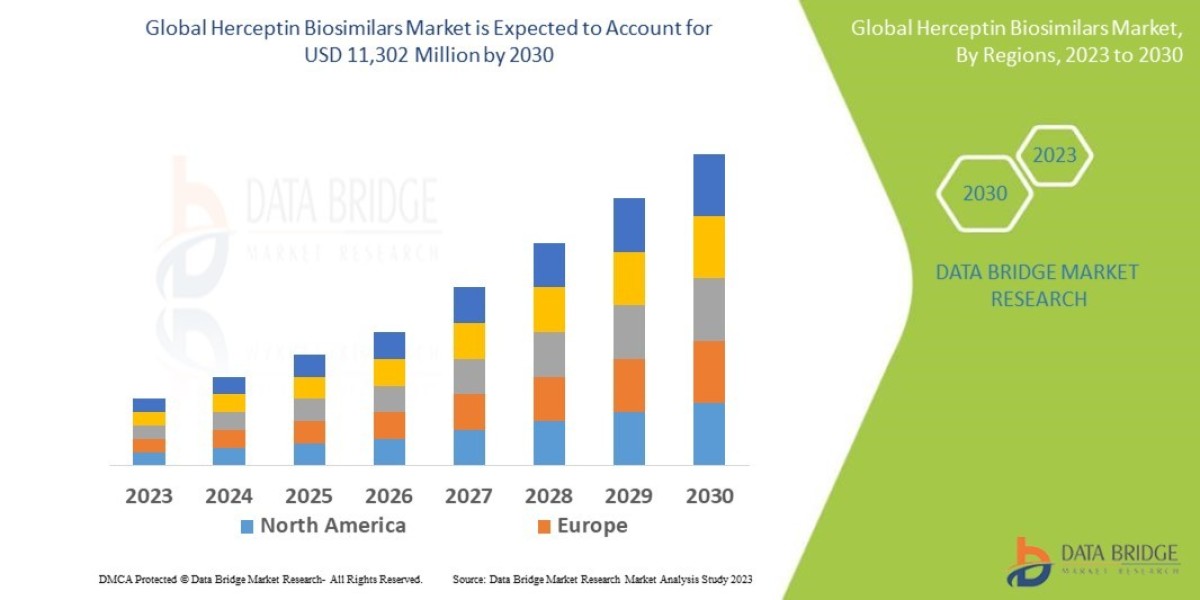
Data Bridge Market Research analyses that the market which was USD 1,810 million in 2022, would rocket up to USD 11,302 million by 2030 and is expected to undergo a CAGR of 23.2% during the forecast period.
Key Factors Driving Growth:
Rising Cancer Incidence:
Increasing global prevalence of HER2-positive breast and gastric cancers fuels demand for cost-effective therapies.Patent Expiry of Herceptin:
Patent expirations in key markets have opened opportunities for biosimilar manufacturers to capture significant market share.Cost-effectiveness of Biosimilars:
Biosimilars offer 20–40% cost reductions compared to originator biologics, enabling healthcare systems to treat more patients within budget constraints.Regulatory Support and Streamlined Approvals:
Regulatory agencies are accelerating biosimilar approvals through harmonized frameworks, promoting global access.Advancements in Biomanufacturing:
Continuous improvements in bioprocessing, purification, and quality assurance have enhanced biosimilar reliability and performance.Growing Investments in Oncology Research:
Public and private funding in oncology biosimilars supports clinical trials, technological innovation, and global expansion.Increased Healthcare Expenditure:
Rising global healthcare budgets, especially in developing economies, create favorable conditions for biosimilar adoption.
Opportunities in Emerging Regions:
Emerging markets in Asia-Pacific, Latin America, and Africa represent high-growth potential due to large patient populations, unmet medical needs, and government-led affordability programs. Strategic partnerships and local manufacturing will be key to unlocking these opportunities.
Conclusion
The Herceptin Biosimilars Market represents a vital component of the global oncology therapeutics landscape. With the growing burden of HER2-positive cancers, biosimilars are poised to play a critical role in expanding access to affordable and effective treatments. Advances in biotechnology, regulatory support, and strategic collaborations are shaping the future trajectory of the market.
Sustainability and innovation will remain core themes as manufacturers balance affordability with quality and safety. The market’s future growth depends on continued investment in R&D, education to build clinical confidence, and policies that support competitive pricing.
By 2035, the Herceptin Biosimilars Market is expected to be a cornerstone of the biosimilar oncology segment, delivering economic and therapeutic value to patients, providers, and healthcare systems worldwide.
Frequently Asked Questions (FAQ)
1. What is the current size of the Herceptin Biosimilars Market?
The global Herceptin Biosimilars Market was valued at around USD 5.4 billion in 2024 and is projected to reach approximately USD 9.8 billion by 2035.
2. What drives the growth of the market?
Key drivers include rising cancer incidence, cost savings of biosimilars, patent expirations, and supportive regulatory frameworks.
3. Which regions dominate the Herceptin Biosimilars Market?
North America and Europe hold significant market shares, while Asia-Pacific is the fastest-growing region due to strong manufacturing and increasing demand.
4. What are the major challenges in the market?
Challenges include high development costs, manufacturing complexity, physician hesitancy, and competitive pricing pressures.
5. What is the projected CAGR of the market from 2025 to 2035?
The market is expected to grow at a CAGR of approximately 6.1% during the forecast period.
6. Who are the primary end-users of Herceptin biosimilars?
Hospitals, oncology clinics, pharmaceutical firms, and research institutions are key end-users.
7. How are biosimilars impacting healthcare affordability?
Biosimilars offer substantial cost savings, allowing healthcare systems to treat more patients and reduce overall oncology treatment expenses.
Browse More Reports:
Global Laminated Labels Market
Global Land Mobile Radio Market
Global Laser Guide Star Adaptive Optics Market
Global Light-Emitting Diode (LED) Phototherapy Equipment Market
Global Leflunomide Market
Global Light Metal Packaging Market
Global Liquid Crystal Electro Optic Modulators Market
Global Liquid Crystal Polymer (LCP) Films and Laminates Market
Global Liquid Particle Counters Market
Global Live Attenuated Vaccines Market
Global Loafers Market
Global Luciferase Assay Kits Market
Global Lung Stent Market
Global Luxury Gin Market
Global Lysosomal Acid Lipase Deficiency Treatment Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com